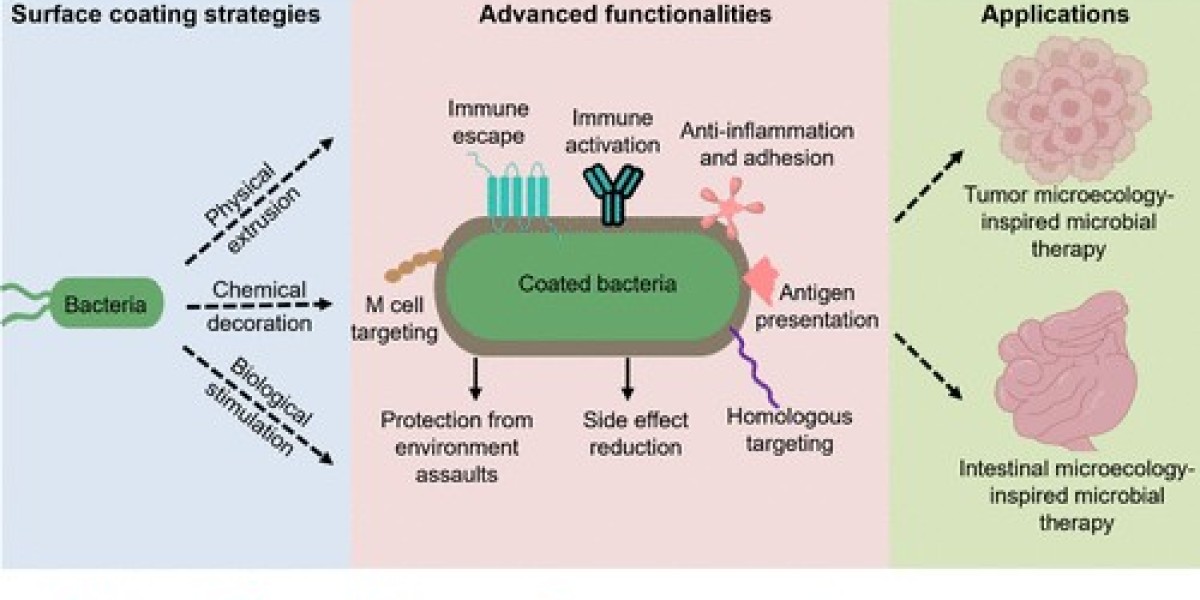
Bacterial protective coverings materials and systems designed to shield useful microbial communities or to prevent harmful bacteria from colonizing surfaces are an emerging cross disciplinary field at the intersection of microbiology, materials science, medicine, and engineering. Innovations in this area address two related but distinct needs: (1) protective coatings that maintain and support beneficial bacteria (for example in probiotics, bioreactors, or agricultural inoculants), and (2) anti-biofouling and antimicrobial surfaces that prevent pathogenic bacteria from attaching, forming biofilms, and causing infections or equipment failure. This overview surveys recent directions, design strategies, practical challenges, and translational pathways for these technologies.
Design principles and material strategies
At the core of any protective covering are three competing design goals: selective permeability (let nutrients in, keep toxins out), mechanical integrity (stability under expected stresses), and biointerface control (encourage or discourage microbial adhesion as desired). Researchers are advancing several material platforms to meet these goals:
Hydrogel matrices and microencapsulation. Hydrogels crosslinked polymer networks that hold water offer a soft, tissue-like environment that can protect bacteria from desiccation and shear while permitting diffusion of nutrients and metabolites. Microencapsulation techniques (alginate beads, gelatin-based microgels, and novel synthetic hydrogels) can create microenvironments for probiotics and industrial microbes, increasing survival during storage and delivery.
Layer-by-layer (LbL) assembled films. LbL assembly builds thin films by alternating charged polymers, proteins, or nanoparticles. This approach enables precise control of thickness, composition, and surface charge important parameters for controlling bacterial adhesion and release of actives such as antimicrobial peptides or growth factors.
Biomimetic extracellular-matrix (ECM) coatings. By imitating natural ECM components collagens, glycosaminoglycans, and adhesive peptides engineered coatings can provide cues that encourage colonization of beneficial bacteria or, conversely, can be modified to resist colonization by pathogens.
Nanocomposite and metal-organic frameworks (MOFs). Incorporating nanoparticles (e.g., silver, copper) or MOFs into coatings provides potent antimicrobial action but requires careful tuning to avoid toxicity to host tissues or beneficial microbes. Emerging work uses controlled-release strategies so antimicrobial effects are localized and time-limited.
Active and smart protective coverings
Traditional passive barriers are being supplemented by intelligent, responsive systems that change behavior in response to environmental cues:
Stimuli-responsive release. Coatings that release antimicrobials, quorum-sensing inhibitors, or enzymes in response to pH changes, bacterial metabolites, or mechanical cues can provide targeted defense only when pathogens attempt colonization—reducing selection pressure for resistance.
Quorum-sensing disruption layers. Instead of killing bacteria outright, some coatings deploy molecules that block quorum sensing (the chemical language bacteria use to coordinate biofilm formation). This anti-virulence approach lowers the chance of resistance development and leaves beneficial microbes largely unaffected.
Electroactive and photocatalytic surfaces. Surfaces that generate reactive species under light or electrical stimulation can actively destroy bacterial cells on demand. Photocatalysts (e.g., titanium dioxide modified for visible light) and electrically conductive coatings integrated into medical devices are areas of active development.
Bacteriophage and probiotic-seeding coatings. Coatings that embed bacteriophages (viruses that infect bacteria) can selectively eliminate targeted pathogens while sparing non-target species. Alternately, surfaces pre-seeded with benign or beneficial bacteria can prevent pathogen colonization by competitive exclusion.
Applications and case studies
Protective coverings span many applications:
Medical devices and implants. Catheters, prosthetic joints, and wound dressings are high-risk surfaces for biofilm-mediated infections. Antimicrobial and anti-adhesive coatings reduce infection rates, and responsive release systems can provide burst protection during the highest-risk window post-implantation.
Food and agriculture. Coatings for seeds and agricultural tools can protect beneficial inoculants during storage and delivery. Encapsulation of plant-growth-promoting bacteria improves shelf-life and field performance.
Industrial bioprocessing. Immobilizing microbes in protective matrices increases stability and reusability of biocatalysts in reactors, allowing continuous processes with lower contamination risk.
Consumer probiotics and pharmaceuticals. Microencapsulation improves survivability of orally delivered probiotics through the stomach acid barrier. In supply-chain contexts, manufacturers and cephalexin capsules distributors alike must understand stability and regulatory expectations for microbe-containing products lessons that are transferrable to microbial covering technologies, especially when antimicrobial agents are involved.
Challenges and safety considerations
Despite promising advances, challenges remain:
Balancing activity with biocompatibility. Antimicrobial-loaded coatings must avoid collateral toxicity to host tissues or disruption of beneficial microbiomes. Dose control and targeted release technologies are essential.
Resistance and ecological impact. Broad-spectrum antimicrobial surfaces risk selecting for resistant strains or disturbing environmental microbial ecology. Approaches that modulate behavior (quorum-sensing interference) or use narrow-spectrum tools (phages or engineered peptides) help mitigate this risk.
Scalability and durability. Lab-scale coatings often rely on precise, time-consuming processes. Translating to large-area industrial coatings or implantable devices requires compatible manufacturing methods, long-term stability, and predictable aging behavior.
Regulatory pathways. When coatings incorporate active pharmaceutical ingredients or living organisms, regulatory requirements become complex. Clear characterization of leachables, long-term safety, and consistent manufacturing are non-negotiable. Early engagement with regulators improves chances of successful translation.
Testing and validation
Robust in vitro and in vivo testing protocols are needed. Standardized biofilm assays, mechanical durability testing, and long-term release profiling are the minimum requirements. Beyond bench tests, real-world simulations flow systems for devices exposed to bodily fluids, soil microcosms for agricultural applications, or continuous bioreactor runs for industrial uses provide critical performance data. Multidisciplinary teams combining microbiologists, materials scientists, clinicians, and process engineers produce the most reliable validation pipelines.
Future directions and outlook
Looking ahead, convergence with synthetic biology and digital manufacturing will accelerate innovation. Designer microbial consortia embedded in protective matrices could perform complex functionsc bioremediation, in situ sensing, or therapeutic delivery while self-regulating through engineered feedback circuits. Additive manufacturing (3D printing) of functional coatings with spatial patterning of active zones will enable device-specific protection. Finally, improved diagnostics for early detection of surface colonization (sensing metabolites, pH shifts) will pair with responsive coatings to create closed-loop defense systems.
Commercialization will require clear value propositions: reduced infection rates, longer device lifetimes, or improved efficacy of probiotic products. Stakeholders across supply chains including manufacturers, distributors, healthcare providers, and patient groups need transparent data on efficacy and safety. For companies that already handle pharmaceuticals and medical supplies, such as cephalexin capsules distributors, attention to packaging, storage conditions, and regulatory compliance for microbial or antimicrobial-containing products will be familiar territory and a helpful foundation for adopting these next-generation surface technologies.
In sum, innovations in bacterial protective coverings are moving from proof-of-concept toward practical deployment across medicine, agriculture, and industry. Success will depend on biologically informed material design, smart responsive behavior, comprehensive safety testing, and careful attention to manufacturability and regulation. When these pieces come together, protective coverings can transform how we manage beneficial microbes and guard against harmful ones making surfaces not simply passive substrates but active participants in microbial control.